Abstract
Protein N-myristoylation is the covalent attachment of myristic acid (C14:0) by an amide linkage to the NH2-terminal glycine residue of numerous eukaryotic and viral proteins, thereafter regulating their membrane targeting or enzymatic activity. A single myristoyl-CoA: protein N-myristoyltransferase (NMT) isoform catalyses the acylation reaction in yeast, insects, plants, and unicellular parasites. It is two separate isoforms however; NMT1 and NMT2, with predominantly overlapping substrates that perform this task in mammals. Myristoylated proteins that fail to undergo the modification are recognised by a dedicated glycine N-degron system and rapidly degraded.
We recently identified a remarkable reduction of NMT2 expression in a wide variety of cancers, especially hematological cancers. As a consequence, we analysed if NMT1/NMT2 could be associated with AML progression. Data analysis from the TCGA transcriptome database showed that high NMT1 and low NMT2 were associated with reduced overall and event-free survival in adult AML, and high NMT1 - but not NMT2 - expression is associated with proliferative gene sets in AML cell lines. One oncogenic myristoylated target identified initially in B-cell lymphoma is the family of Src kinases (SFKs). Here, we demonstrate that PCLX-001 significantly reduces total and phosphorylated SFKs, which are essential for pro-survival signalling downstream frequently mutated c-Kit and FLT3 receptors in multiple AML cell lines. Moreover, inhibiting myristoylation led to ER stress induction and apoptosis in cell lines and patient blast samples at concentrations sparing most peripheral blood lymphocytes and monocytes from healthy individuals.
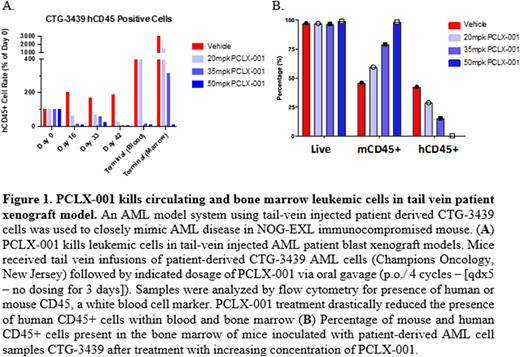
We further validated the therapeutic potential of our lead myristoylation inhibitor ongoing clinical trial evaluation against lymphoma and solid tumours (PCLX-001) in a variety of AML xenograft models. PCLX-001 monotherapy had a dose-dependent tumoricidal effect in an ectopic sub-cutaneous MV-4-11 xenograft model, in two tail vein injected patient derived xenografts (PDXs) as well as in 3 out of 5 intra-femoral PDXs. Of note, five days of PCLX-001 treatment at the highest concentration was sufficient to obtain complete tumour eradication in our CDX model. In tail-vein injected PDX models, PCLX-001 treatment resulted in up to 95% reduction of human CD45+ cells in peripheral blood and bone marrow with minimal toxicity or long-term impact on body weight (Fig.1). Further analyses are required to characterize the cytogenetics, common mutations or NMTs expression profile that could explain the sensitivity of certain patient models compared to the others.
An ongoing challenge in treatment of leukemia resides in leukemic stem cells (LSCs), a low-frequency sub-population functioning as progenitor cells. Without eradication of LSCs no patient can be cured. Relapse rates are high in AML and are often featuring additional mutations and drug resistance. Here, we demonstrate that LSC subpopulations (CD34+/CD38- and CD117+/ CD15-) in the OCI-AML-22 patient derived model are exquisitely sensitive to PCLX-001 treatment in vitro and began shrinking after 48 hours of treatment. In addition, the LSC subpopulation showed lower IC50 compared to the general cell population at 72 hours. Interestingly, we identified that functionally validated LSC+ patient subpopulations were expressing lower amount of NMT2 compared to LSC-. This observation could partially explain the sensitivity of the LSC subpopulation.
In aggregate, our preclinical observations suggest that PCLX-001 is highly potent against AML cells in vitro and in vivo (both circulating and in the bone marrow), and, that LSCs are also more sensitive to PCLX-001 than non-leukemic stem cells. This further supports the initiation of PCLX-001evaluation in AML clinical trials scheduled to start in late 2022.
Disclosures
Gamma:Pacylex Pharmaceuticals: Current equity holder in private company. Beauchamp:Pacylex Pharmaceuticals: Current Employment, Current equity holder in private company. Yap:Pacylex Pharmaceuticals: Current equity holder in private company. Brandwein:Abbvie: Honoraria; BMS/Celgene: Honoraria; Taiho: Honoraria; Pfizer: Honoraria; Astellas: Honoraria; Amgen: Honoraria; Jazz: Honoraria; Merck: Honoraria. Wang:Trillium Therapeutics: Patents & Royalties; Pfizer: Patents & Royalties. Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. Borthakur:Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy. Mackey:Pacylex Pharmaceuticals, Inc.: Current equity holder in private company. Berthiaume:Pacylex Pharmaceuticals, Inc.: Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal